Introduction
Hydroxyurea (HU), standard therapy for children and adults with severe sickle cell disease (SCD) in resource-rich countries, reduces the frequency of vaso-occlusive complications, increases hemoglobin, and decreases mortality. While guidelines recommend escalating HU to maximum tolerated dose (MTD), use of fixed low and moderate doses of HU are common in low resource countries. HU at MTD is superior to fixed moderate-dose HU in decreasing vaso-occlusive complications, hospitalizations, and blood transfusions in young children with SCD but has not been compared with fixed low or moderate doses in adults. We conducted a systematic review and meta-analysis to evaluate the efficacy of escalated dose HU versus fixed low-dose HU in adults with SCD.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA), to evaluate if clinical and laboratory outcomes differ between SCD patients treated with HU administered with an escalated versus fixed low-dose strategy. Primary outcomes included vaso-occlusive crisis (VOC), acute chest syndrome, hospitalizations, blood transfusions, and stroke. Secondary outcomes included hemoglobin concentration, fetal hemoglobin (HbF), absolute neutrophil count (ANC), reticulocyte count, and platelet count.
Literature search was conducted in PubMed, Embase, Cochrane, and Scopus and included peer-reviewed quantitative studies published from 1995 to 2020. Abstracts and full text articles were reviewed to identify articles that met inclusion criteria. Following the initial search, 2524 citations were retrieved and screened using Rayyan, a web-based software designed to conduct and coordinate systematic literature reviews. After eliminating 385 duplicates, 2139 citations remained. Seventeen full-text articles were then assessed for eligibility, of which 10 were included in the quantitative synthesis. Blinded co-authors used Rayyan to independently check the titles and abstracts based on pre-decided exclusion criteria. This was followed by a full text review of selected articles, and any conflicts were resolved by consensus. Data were systematically extracted using a standardized spreadsheet customized for the study. Average effects across studies were estimated using means for fixed-effect meta-analysis. We calculated the difference in means: MD i = m 1 i - m 2 i with standard errors. The significance of the overall effect (escalated and fixed low-dose) combined was tested using Z tests and group differences between escalated and fixed doses were compared using chi-squared tests. Analyses were conducted in Review Manager (RevMan) version 5.3.
Results
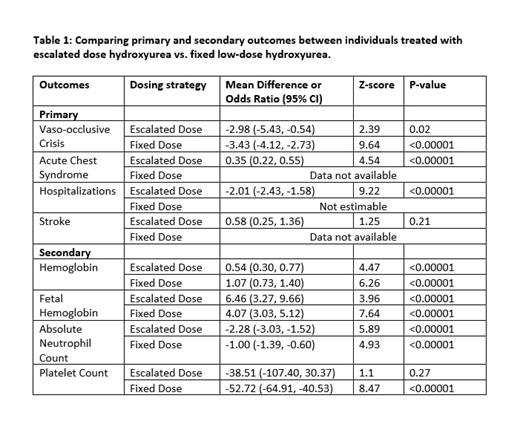
Ten studies were included in the quantitative synthesis - 5 evaluated the effect of HU at escalated dose and 5 evaluated fixed low-dose HU. The average daily doses of HU in the low fixed-dose and escalated dose studies were ~10 mg/kg and 22 mg/kg, respectively. Available primary and secondary outcome data for HU at escalated and fixed low-doses from eligible studies are summarized in Table 1.
There was no difference in the estimate of VOC crisis rate between the escalated dose and fixed low-dose studies (p=0.73). The mean difference in hemoglobin from baseline to follow-up was greater for fixed low-dose than escalated dose studies (1.07 g/dL vs. 0.54 g/dL, p=0.01). The mean decrease in ANC from baseline to follow-up was greater for escalated dose than low fixed-dose studies (-2.28 [x10 9/L] vs. -1.00 [x10 9/L], p=0.003). No difference was seen in the mean estimate of fetal hemoglobin between escalated dose and fixed low-dose studies.
Conclusions
Meta-analysis of escalated and fixed low-dose HU showed no significant differences in VOC rate, although the mean difference in hemoglobin was greater for fixed low-dose studies. Comparison of effects between escalated and fixed low-dose HU on acute chest syndrome, hospitalizations, blood transfusion and stroke were not performed due to lack of data. Based on these limited findings, there appears to be clinical equipoise regarding the most appropriate HU dosing regimen to decrease vaso-occlusive complications in adults with SCD. A controlled clinical trial comparing escalated versus low fixed-dose HU in adults with SCD is necessary in low- and middle-income countries where fixed low-dose HU is commonly administered.
Disclosures
Ogu:Vertex Pharmaceuticals: Consultancy; Bluebird Bio: Consultancy; Emmaus: Speakers Bureau; Global Blood Therapeutics/Pfizer: Speakers Bureau. Ataga:Fulcrum Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Hillhurst Biopharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; FDA: Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; NHLBI: Research Funding; Roche: Consultancy, Honoraria; Biomarin: Consultancy, Honoraria; Vertex: Other: Data Monitoring Committee; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceuticals: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal